1. All the commercial liquid fuels are derived from natural petroleum (or crude oil).
A. True
B. False
2. A cycle consisting of one constant pressure, one constant volume and two isentropic processes is known as
A. Carnot cycle
B. Stirling cycle
C. Otto cycle
D. Diesel cycle
3. The efficiency and work ratio of a simple gas turbine cycle are
A. low
B. very low
C. high
D. very high
4. The amount of heat required to raise the temperature of the unit mass of gas through one degree at constant volume is called
A. specific heat at constant volume
B. specific heat at constant pressure
C. kilo Joule
D. none of these
5. There is a loss of heat in an irreversible process.
A. True
B. False
6. An adiabatic process is one in which
A. no heat enters or leaves the gas
B. the temperature of the gas changes
C. the change in internal energy is equal to the mechanical workdone
D. all of the above
7. Water gas is obtained by passing air and a large amount of steam over waste coal at about 650°C.
A. Correct
B. Incorrect
8. Which of the following represents Otto cycle on temperature – entropy (T – s) diagram?
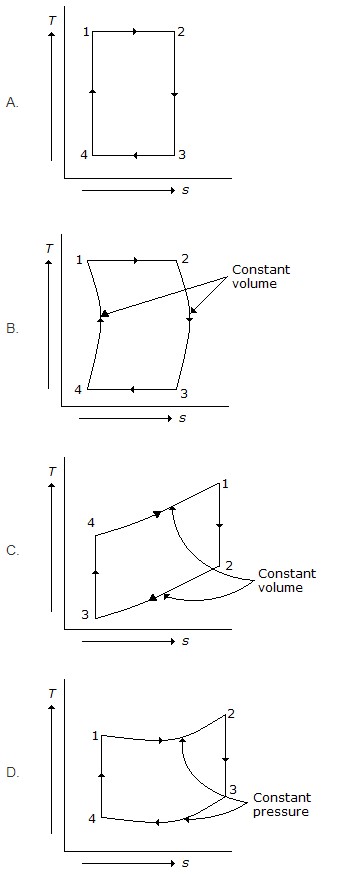
9. When the gas is heated at constant volume, the heat supplied increases the internal energy of the gas.
A. True
B. False
10. Which of the following is the lightest and most volatile liquid fuel?
A. Gasoline
B. Kerosene
C. Fuel oil