11. The processes occurring in an open system which permit the transfer of mass to and from the system, are known as
A. flow processes
B. non-flow processes
C. adiabatic processes
D. none of these
12. Which of the following has the minimum atomic mass?
A. Oxygen
B. Sulfur
C. Nitrogen
D. Carbon
13. Workdone in a free expansion process is
A. zero
B. minimum
C. maximum
D. positive
14. The pressure exerted by an ideal gas is __________ of the kinetic energy of all the molecules contained in a unit volume of gas.
A. one-half
B. one-third
C. two-third
D. three-fourth
15. The compression ratio for petrol engines is
A. 3 to 6
B. 5 to 8
C. 15 to 20
D. 20 to 30
16. The most probable velocity of the gas molecules is given by
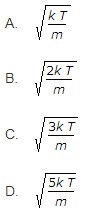
17. The efficiency of Diesel cycle approaches to Otto cycle efficiency when
A. cut-off is increased
B. cut-off is decreased
C. cut-off is zero
D. cut-off is constant
18. The entropy __________ in an irreversible cyclic process.
A. remains constant
B. decreases
C. increases
19. The atomic mass of oxygen is
A. 12
B. 14
C. 16
D. 32
20. The ratio of specific heat at constant pressure (cp) and specific heat at constant volume (cv) is
A. equal to one
B. less than one
C. greater than one
D. none of these